Trial Requirements
We are inviting you to take part in a clinical trial.
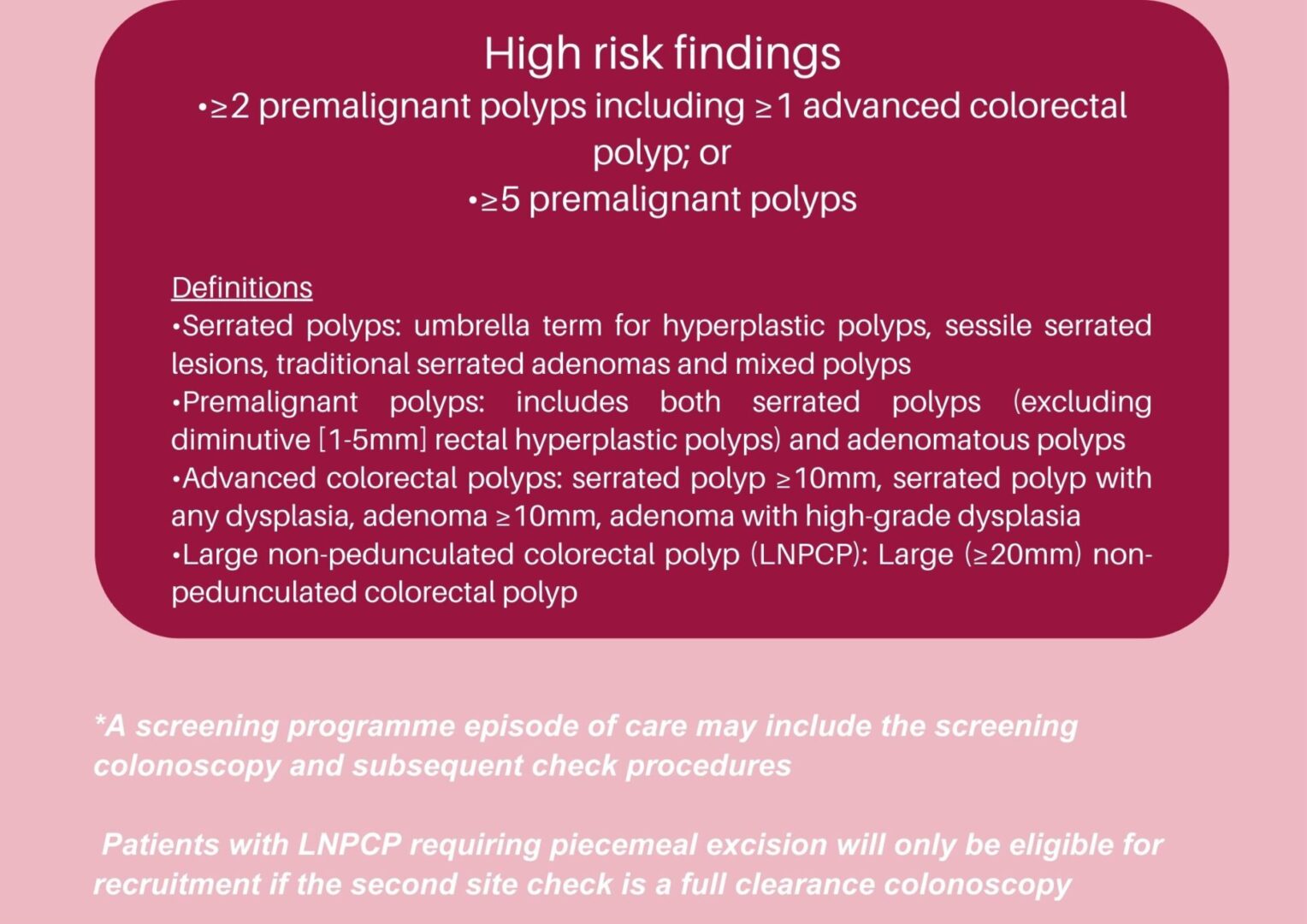
High risk definition
The Figure below shows the eligibility criteria for participants identified as high risk through the BCSP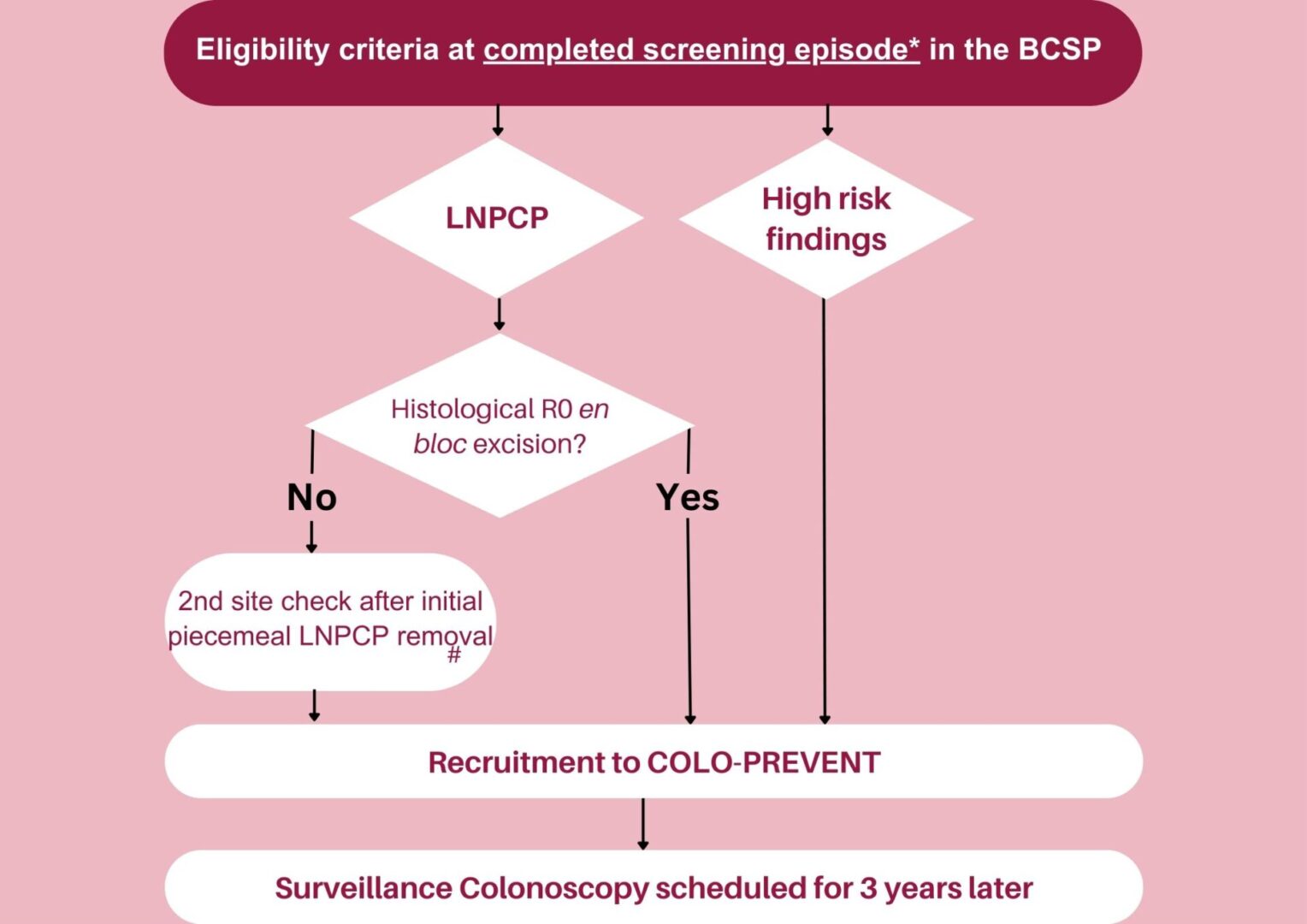
Eligibility criteria
Inclusion criteria
General inclusion criteria for both trials:
• Adequate renal function, defined as GFR ≥35ml/min/1.73m2, at any time in the preceding 4 weeks
• Willing and able to consent to participate in trial
Participants must meet ONE of the following criteria:
• Patients with high risk findings (≥2 premalignant polyps
including ≥1 advanced colorectal polyp; or ≥5
premalignant polyps) at a completed screening episode
according to BCSP criteria OR
• Patients with a large (≥20mm) non-pedunculated colorectal polyp that is resected with histological R0 en bloc excision
at a completed screening episode OR
• Patients with a large (≥20mm) non-pedunculated colorectal polyp after piecemeal excision. These will only be eligible if the subsequent 2nd site check is a full clearance
colonoscopy
Inclusion criteria for the main trial but not the resveratrol Signal-Seeking trial:
• Aged 50-71 years
Additional inclusion criteria for the resveratrol Signal-Seeking trial only:
• Aged 50-73 years
• Use of aspirin, including as an anti-platelet therapy, is
permitted in the signal-seeking trial
Exclusion criteria
General exclusion criteria:
- Malignant change in a polyp
- Known clinical diagnosis or gene carrier of a hereditary CRC predisposition (FAP, hereditary nonpolyposis CRC)
- Previous or newly diagnosed inflammatory bowel disease
- Previous or planned colorectal resection
- Known bleeding diathesis or concomitant non-aspirin anti-coagulant or anti-platelet agent
- Abnormal liver functions consisting of any of the following, at any time in the preceding 4 weeks:
- Serum bilirubin ≥1.5 x ULN (except for participants with Gilbert’s disease, for whom the upper limit of serum bilirubin is 51.3μmol/l or 3mg/dl)
- Aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥2.5 x ULN
- Inability to comply with trial procedures and use of therapies
- Pregnant or lactating women. Women of child-bearing potential must agree to use appropriate methods of birth control (see protocol section 8.10)
- Males with partners who are WOCBP and are unwilling to use effective methods of contraception
- Serious medical illness interfering with trial participation including inability to have future colonoscopic surveillance
- Participants who have been administered an investigational medicinal product for another research trial in the last 30 days or ≤5 elimination half-lives
Exclusion criteria:
- Regular (>3 doses per week) prescribed or ‘over the counter’ (OTC) aspirin or regular (>3 doses per week) prescribed or OTC non-aspirin NSAID use
- Allergic or intolerant to ibuprofen or naproxen, metformin, aspirin or salicylate
- Diabetic patients on drug treatment
- Current or previous treatment with metformin
- Known history of peptic ulcer disease
- Known history of lactic acidosis or predisposing conditions
- Prior use of NSAIDs is not an exclusion if they are self-prescribed and the patient is willing to stop use for the duration of the trial
- Use of long-term systemic corticosteroids
Endpoints and outcome measures
Primary endpoint/outcome
Polyp number measured by MPP (Mean number of Polyps per Participant)
Secondary endpoints/outcomes
• Polyp Detection Rate (PDR, proportion of individuals with one or more qualifying* pre-malignant polyp(s)at surveillance)
• Advanced polyps (measured as MPP and PDR); these are defined as serrated polyp ≥10mm, serrated polyp with any dysplasia, adenoma ≥10mm, adenoma with high-grade dysplasia
• Polyp subtype based on histopathology (adenoma/serrated); also reported as MPP and PDR
• Location of polyps (right colon - any part of the colon proximal to the splenic flexure; left colon – the rectum and the colon distal to and including the splenic flexure)
• Polyp size (maximum dimension in mm as described in the histopathology report or endoscopic size if no histopathological size available)
Essential Resource
COLO-PREVENT Protocol Summary & Schedule
COLO-PREVENT MAIN Trial Patient Information Sheet
COLO-PREVENT MAIN Trial Consent Form
COLO-PREVENT Site Feasibility Questionnaire
YouTube FFQ (Food Frequency Questionnaire) help for sites and patients on how to complete the questionnaire:
The paper Main Trial FFQ can be found here:
The paper Resveratrol Sub-Trial FFQ can be found here:
How To Report A Serious Adverse Event (SAE)
All Serious Adverse Events MUST be reported within 24 hours of the research team becoming aware of the event. The initial report may be submitted without causality/expectedness section completed or a PI/delegated medic signature, but this must be followed up with a signed copy reporting expectedness and causality within 7 days.
Once a signed initial report is received, a follow up or final report should be submitted within 28 days. The reporting person(s) will receive an ‘acknowledgment of receipt’ email from the Sponsor following the submission of each report. This will contain the date by which a follow-up or final report should be submitted, and details of any additional queries raised. Response to the request is required as per the timelines dictated in the email. If the participant is still an inpatient, or there is an unavoidable delay in the provision of further information, inform the Sponsor at the Research Governance Office.
Please return the completed COLO-PREVENT SAE Reporting Form A Including Cover Sheet and any anonymised copies of supporting documents to [email protected] and Leicester CTU [email protected]
For more information on how to complete this form please refer to the Sponsor Guidance document found here.
Investigator sites map
Contact us
If you would like more information, would like to express interest as an NHS trust or you are considering joining the trial, please get in touch.

